
Captive breeding of the Egyptian tortoise Testudo kleinmanni. A C Highfield & Jill Martin
The Egyptian tortoise is one of the least known Mediterranean tortoises, both in terms of its natural history and its captive maintenance. Breeding outside its natural zone of distribution has rarely been recorded.
Description
The unfamiliarity of this species to many authorities has resulted in a number of errors in previous publications relating to basic diagnostic characters and descriptions. As there is so much misinformation on this topic in print, it is worth reviewing the true facts:
Plastron: The plastron of the vast majority of specimens features two 'V' shaped brown or black markings upon the abdominal scutes. The only other Mediterranean tortoise with similar markings is the much large Testudo marginata. This feature is present on all but very few individuals and is quite different from the abdominal marks seen on the plastron of 'graeca' complex tortoises. The authors have seen a small number of kleinmanni from Libya with entirely unmarked plastrons, but these are atypical of kleinmanni as a whole. The ground of the plastron is pale straw-coloured.
Carapace: The ground of the carapace ranges from extremely pale dull yellow to bright straw coloured with a hint of yellowish-green. The anterior and lateral margins of the vertebral scutes are delineated in dark brown to black. The anterior and external lateral margins of the costals are similarly distinguished. In some specimens these markings are much diminished. The centres of the scutes are unmarked and lack the typical dark aureole of 'graeca' complex animals.
Skin coloration: The skin of the head and limbs are very pale yellow to ivory colour. Again, the only other Mediterranean tortoise to feature similar skin coloration is Testudo marginata. In marginata, however, the head is dark brown to black and is much more elongate in form than that of kleinmanni.
Unlike Testudo graeca, Testudo kleinmanni lacks thigh tubercles. The tail also lacks a terminal claw (as seen in T. hermanni, for example) although several published diagnostic keys have erroneously asserted that it does possess this feature. The front limbs of kleinmanni also feature greatly enlarged imbricate scales compared to T. graeca. This feature is very obvious even on newly hatched specimens. The most obvious distinguishing characteristic of Testudo kleinmanni, however (compared to T. graeca) is its remarkably diminutive size. Males are smaller than females, and have an average Straight Carapace Length of only 95mm. The largest female recorded had an SCL of only 127mm.
Captive environment
Our T. kleinmanni accommodation was developed in something of a rush, however, it has proved so successful that we have retained the basic set-up for the past 12 months and although we intend to develop it further, we do not envisage any drastic changes. Each unit measures 2' X 6' and is further sub-divided into two to allow separation of males and females. It consists of a plywood base surrounded by a 6" high coated chipboard wall. Lighting is simple in the extreme, consisting of a single 40W mini-spot light situated in each section. A 40 W Tru-lite full spectrum tube is also included. Each section features a potted plant (for decoration and shade) plus a number of rocks to encourage climbing activity and a 60% dry loam 40% sand substrate. Overnight heating is provided by low-wattage heat pads. Ambient daytime temperatures are maintained in the region of 17-24C with most activity occurring in the middle of this range. It is a common error of kleinmanni husbandry to employ ambient temperatures which are far too high. At higher temperatures (30°C and above) activity decreases and under natural conditions the tortoises will aestivate. It is difficult to replicate conditions for safe aestivation in captivity, and for this reason our specimens are maintained at moderate temperatures which permit all-year round activity.
Diet
The dangers inherent in high protein foods for Mediterranean tortoises have been understood for some time. In accordance with these principles, our T. kleinmanni are maintained on a 100% vegetable diet with as high a proportion of wild-picked foods (such as clover and dandelion) as practicable. The overall balance of the diet is geared towards a high fibre, low protein and high calcium intake. No fruit is provided (other than the occasional piece of tomato) and water is available at all times if required. Calcium and D3 is provided by means of Nutrobal or Rep-Cal sprinkled liberally on each daily feed. Provided D3 is available via oral supplementation our experience over many years with a variety of species suggests that it is not necessary to resort to high UV output lighting systems. Our aim is for slow growth, approaching natural rates, and we specifically avoid overfeeding - a common but often unrecognised failing of many captive breeding programs.
Breeding behaviour
The most remarkable feature of breeding behaviour in T. kleinmanni concerns vocalisation: both male and female kleinmanni are very vocal compared to most tortoises and the males in particular produce a sound not unlike a dove calling during mating. Considering the diminutive size of the tortoises, the volume produced is astonishing! This sound is quite unlike that of any other Mediterranean tortoise. Amorous males circle females, do not appear to engage in biting behaviour, but do ram the female's shell, possibly in an attempt to cause her to remain static. Mating often lasts 20 minutes or more. Typically, males do not show interest in mating when females are carrying eggs.
Egg laying
In the wild, T. kleinmanni nests are excavated to a depth of 3-5 cm in a sandy substrate. In captivity, our indoor unit allowed an excavation depth of no more than 2.5 cm but this did not seem to inhibit laying. One egg was laid in an outdoor sand-pit on a particularly hot summer's day. The eggs are elongate and range from 31.5 mm long X 22.5 mm wide to 34 mm long x 24mm wide with an average of 32.5mm long X 23.5mm wide. The eggs weigh between 7-9 g. Females typically lay one egg at a time, but on occasions two are laid simultaneously. Large females may lay up to 4 eggs simultaneously, but this is not common. Laying normally occurs at monthly intervals, and continues until a "clutch" of 4 to 5 eggs has been deposited. This is then followed by two to four months without laying during which males show increased interest in mating.
Incubation
A standard static air incubator is used for all hard-shelled Testudo eggs. Temperature control is by means of a proportional controller which operates on a cyclic basis, avoiding the sudden fluctuations common to normal on-off thermostats. This type of controller has proved highly accurate and is extremely durable: the incubator used for the kleinmanni eggs was built in 1986 and has been in daily use ever since. A small industrial drying cabinet heater, rated at 60 Watts is situated in the base of the unit and the eggs are placed in trays on a shelf above the heater. For Testudo and Geochelone eggs (unlike soft-shelled aquatic turtle eggs) the incubation substrate does not seem at all critical. Sandy earth, vermiculite or a combination of both have been used with equal success. The eggs rest on the surface of the substrate and are not buried. Humidity within the incubator is provided by means of a water tray containing a sponge. For Testudo eggs the relative humidity is normally maintained at 70-80%, but again this does not seem critical.
Care of juveniles
Diet and environmental temperature is as for adults. Due to their extremely small size and fragility we place neonate kleinmanni in a covered, but well ventilated, seed-tray propagator. Some gentle base heating is provided, in addition to radiant heat from a basking lamp suspended above the propagator. For the first few weeks of life a paper towel substrate is employed. As the juveniles grow, they can be removed to a smaller version of the adult accommodation.
![]() Although animals may be appear in dealer's lists from time to time, these are almost invariably illegally exported wild-caught specimens. Seizures of illegal specimens have, however, provided the core stock of several specialist collections including those of Jersey Wildlife Preservation Trust who achieved the first recorded European captive breeding of this species in 1990, and the Tortoise Trust who achieved what is believed to be the first mainland British captive breeding in November 1994.
Although animals may be appear in dealer's lists from time to time, these are almost invariably illegally exported wild-caught specimens. Seizures of illegal specimens have, however, provided the core stock of several specialist collections including those of Jersey Wildlife Preservation Trust who achieved the first recorded European captive breeding of this species in 1990, and the Tortoise Trust who achieved what is believed to be the first mainland British captive breeding in November 1994.
![]() Our T. kleinmanni stock currently includes 7 adults; 3 females and 4 males. One female has been in our care for over 3 years, the remainder joined our collection in September and October 1993. The captive breeding success reported here followed a mating between our original female and one of the newly acquired males.
Our T. kleinmanni stock currently includes 7 adults; 3 females and 4 males. One female has been in our care for over 3 years, the remainder joined our collection in September and October 1993. The captive breeding success reported here followed a mating between our original female and one of the newly acquired males.
![]() Of critical importance in the husbandry of T. kleinmanni is the need to prevent cross infection from other tortoises; this species is extremely sensitive to "alien" disease organisms and under no circumstances should be allowed contact with other species. Our own colony is maintained in strict isolation. If contact is permitted, expect serious respiratory and gut parasite problems. In such a small animal, these may rapidly prove fatal.
Of critical importance in the husbandry of T. kleinmanni is the need to prevent cross infection from other tortoises; this species is extremely sensitive to "alien" disease organisms and under no circumstances should be allowed contact with other species. Our own colony is maintained in strict isolation. If contact is permitted, expect serious respiratory and gut parasite problems. In such a small animal, these may rapidly prove fatal.
![]() Feeding foods which are excessively high in protein can certainly produce lumpy shells (and lead to long-term renal problems) but so can feeding too much of the "right" type of food - in the wild many tortoises enjoy a very cyclic feeding pattern, with poor food availability for much of the season due to extreme heat and aridity. In captivity many keepers tend to provide too much of a good thing.
Feeding foods which are excessively high in protein can certainly produce lumpy shells (and lead to long-term renal problems) but so can feeding too much of the "right" type of food - in the wild many tortoises enjoy a very cyclic feeding pattern, with poor food availability for much of the season due to extreme heat and aridity. In captivity many keepers tend to provide too much of a good thing.
![]() The normally suggested incubation temperature for most Testudo species is circa 30-31°C, and at this temperature T. hermanni, T. marginata and T. graeca will usually hatch in 8-9 weeks. Climatic and research data, however, suggests that in the case of T. kleinmanni a higher average temperature is more appropriate. Our incubator was adjusted to provide a constant 33°C, a temperature which in Testudo graeca would typically produce all-female neonates, although for a period of 28 days during the middle-third period in the estimated incubation cycle, we raised this temperature to 35°C to approach peak temperatures experienced under natural conditions.
The normally suggested incubation temperature for most Testudo species is circa 30-31°C, and at this temperature T. hermanni, T. marginata and T. graeca will usually hatch in 8-9 weeks. Climatic and research data, however, suggests that in the case of T. kleinmanni a higher average temperature is more appropriate. Our incubator was adjusted to provide a constant 33°C, a temperature which in Testudo graeca would typically produce all-female neonates, although for a period of 28 days during the middle-third period in the estimated incubation cycle, we raised this temperature to 35°C to approach peak temperatures experienced under natural conditions.
![]() Jersey Zoo's 1990 hatchlings were incubated at a constant 30°C, and hatched in 85 to 95 days. All five hatchlings proved to be males. Our two present hatchlings emerged in 78 days and 111 days respectively, with two more apparently viable eggs yet to hatch. 1 egg fractured prematurely and an almost fully developed neonate unfortunately failed to survive. In the wild, hatching can occur in as little as 70 days. Our prime objective here, if possible, was to produce females. Although no specific ESD (Environmental Sex Determination) data is available for Testudo kleinmanni, it seemed reasonable to assume that an increase in temperature would predispose the eggs towards female young.
Jersey Zoo's 1990 hatchlings were incubated at a constant 30°C, and hatched in 85 to 95 days. All five hatchlings proved to be males. Our two present hatchlings emerged in 78 days and 111 days respectively, with two more apparently viable eggs yet to hatch. 1 egg fractured prematurely and an almost fully developed neonate unfortunately failed to survive. In the wild, hatching can occur in as little as 70 days. Our prime objective here, if possible, was to produce females. Although no specific ESD (Environmental Sex Determination) data is available for Testudo kleinmanni, it seemed reasonable to assume that an increase in temperature would predispose the eggs towards female young.
![]() One interesting observation on kleinmanni incubation is that both embryonic development and the incubation period is very unpredictable. Periods of arrested development (diapause) seem commonplace. We have certainly noted that development is usually extremely slow for the first month to 6 weeks, then accelerates rapidly. In one instance, 3 months passed before any noticeable development occurred.
One interesting observation on kleinmanni incubation is that both embryonic development and the incubation period is very unpredictable. Periods of arrested development (diapause) seem commonplace. We have certainly noted that development is usually extremely slow for the first month to 6 weeks, then accelerates rapidly. In one instance, 3 months passed before any noticeable development occurred.
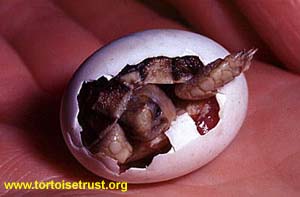
![]() Following initial "pipping" of the egg, the neonates may remain within the egg for several days whilst any remaining yolk-sac is fully absorbed. On emergence, the hatchlings measured between 28-30mm in length and weighed between 4-5 g. They drank water from a shallow dish almost immediately, and began feeding upon desiccated dandelion leaves within 12 hours.
Following initial "pipping" of the egg, the neonates may remain within the egg for several days whilst any remaining yolk-sac is fully absorbed. On emergence, the hatchlings measured between 28-30mm in length and weighed between 4-5 g. They drank water from a shallow dish almost immediately, and began feeding upon desiccated dandelion leaves within 12 hours.
![]() In summary, the threatened status of this species in the wild emphasises the need to develop reliable captive breeding techniques which can be employed to maximise the productivity of stocks now in captivity and in future, hopefully, to reinforce depleted natural populations.
In summary, the threatened status of this species in the wild emphasises the need to develop reliable captive breeding techniques which can be employed to maximise the productivity of stocks now in captivity and in future, hopefully, to reinforce depleted natural populations.